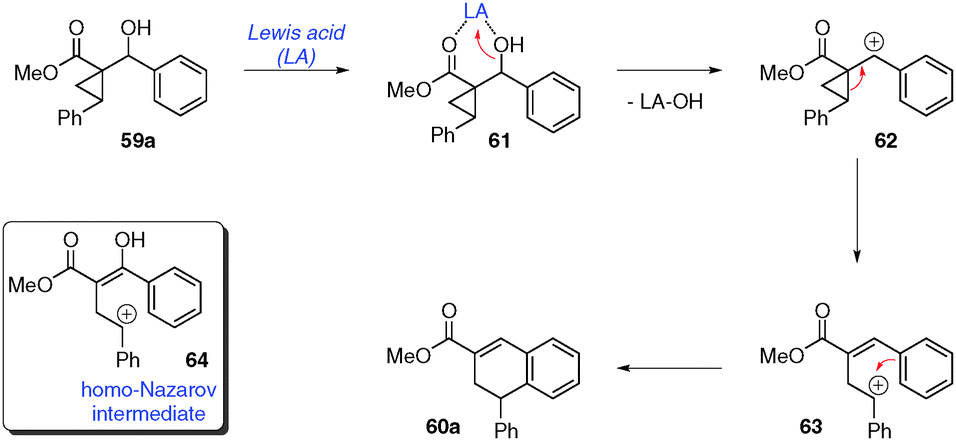
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A

Stress relief: Exercising Lewis acid catalysis for donor-acceptor cyclopropane ring-opening annulations, a basis for new reaction methodologies | Semantic Scholar
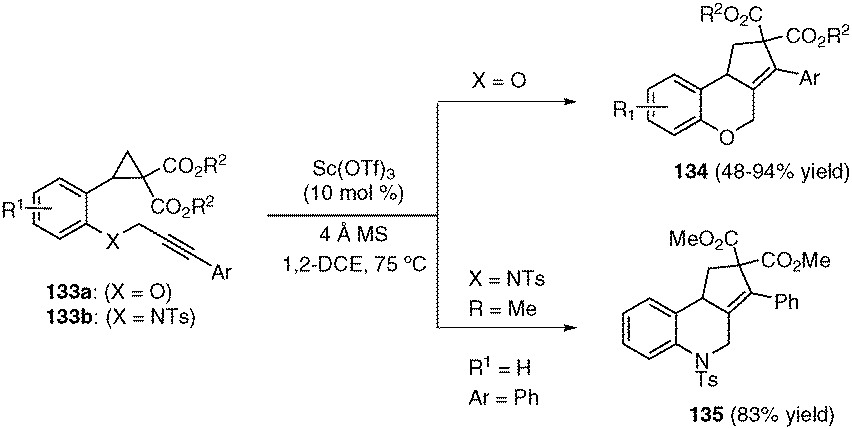
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A
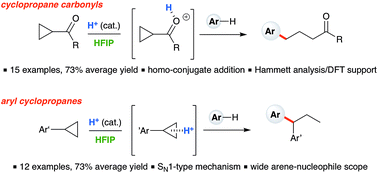
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing)

Recent Advances in the Chemistry of Doubly Activated Cyclopropanes: Synthesis and Reactivity | Bentham Science

Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol. - Abstract - Europe PMC

Ring opening of cyclopropylcarbinyl radicals resulting from hydrogen... | Download Scientific Diagram

Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol. - Abstract - Europe PMC
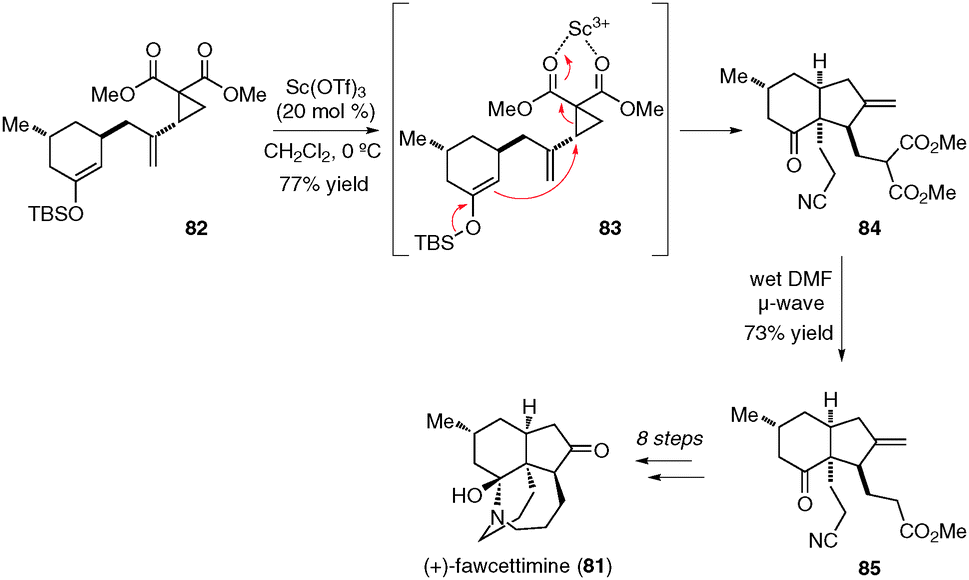
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A
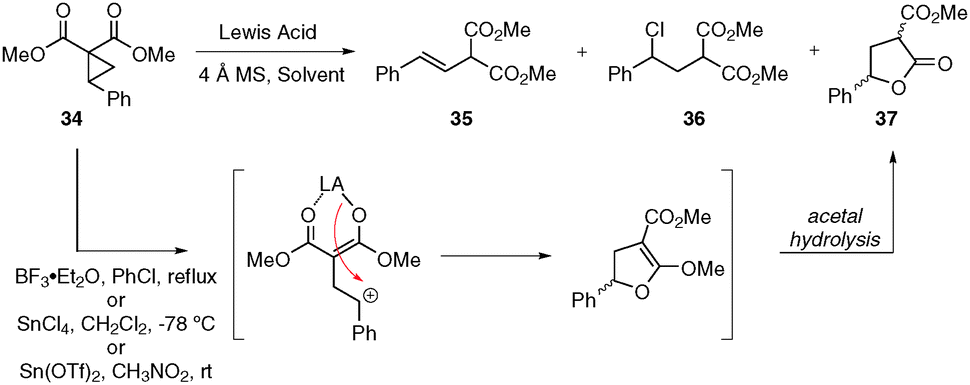
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A

Dearomative Cyclopropanation of Naphthols via Cyclopropene Ring‐Opening - Baker - - Angewandte Chemie International Edition - Wiley Online Library

Mild Ring‐Opening 1,3‐Hydroborations of Non‐Activated Cyclopropanes - Wang - 2018 - Angewandte Chemie International Edition - Wiley Online Library
![Metal‐Free Ring Opening Cyclization of Cyclopropane Carbaldehydes and N‐Benzyl Anilines: An Eco‐Friendly Access to Functionalized Benzo[b]azepine Derivatives - Dey - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library Metal‐Free Ring Opening Cyclization of Cyclopropane Carbaldehydes and N‐Benzyl Anilines: An Eco‐Friendly Access to Functionalized Benzo[b]azepine Derivatives - Dey - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/843b5375-d568-49d5-9e1f-3a3aeb92b0b6/adsc201801714-toc-0001-m.jpg)






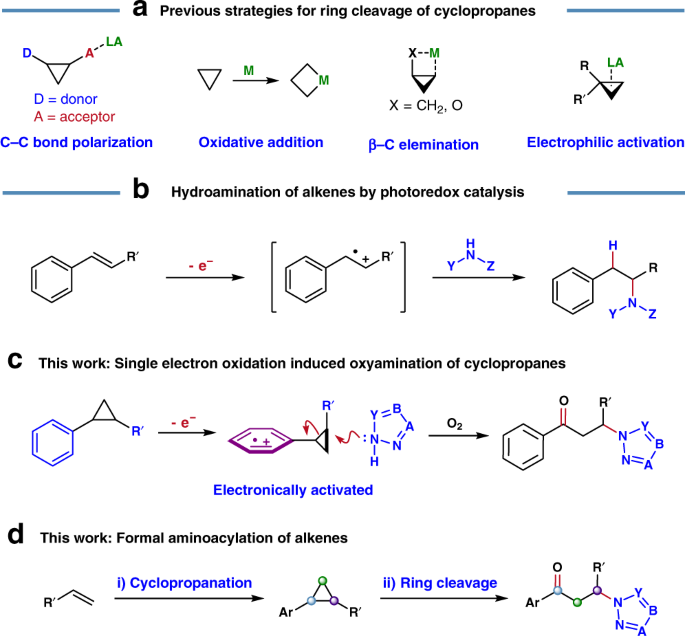




/chapter1/pages25and26/page25and26_files/cyclomech.png)
