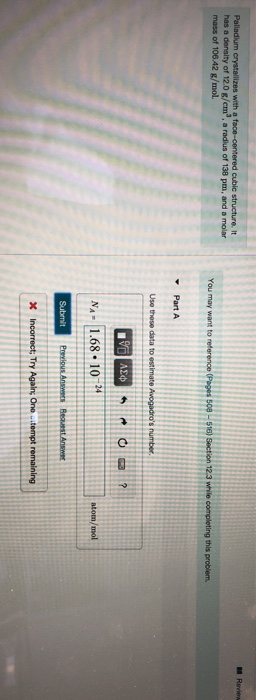
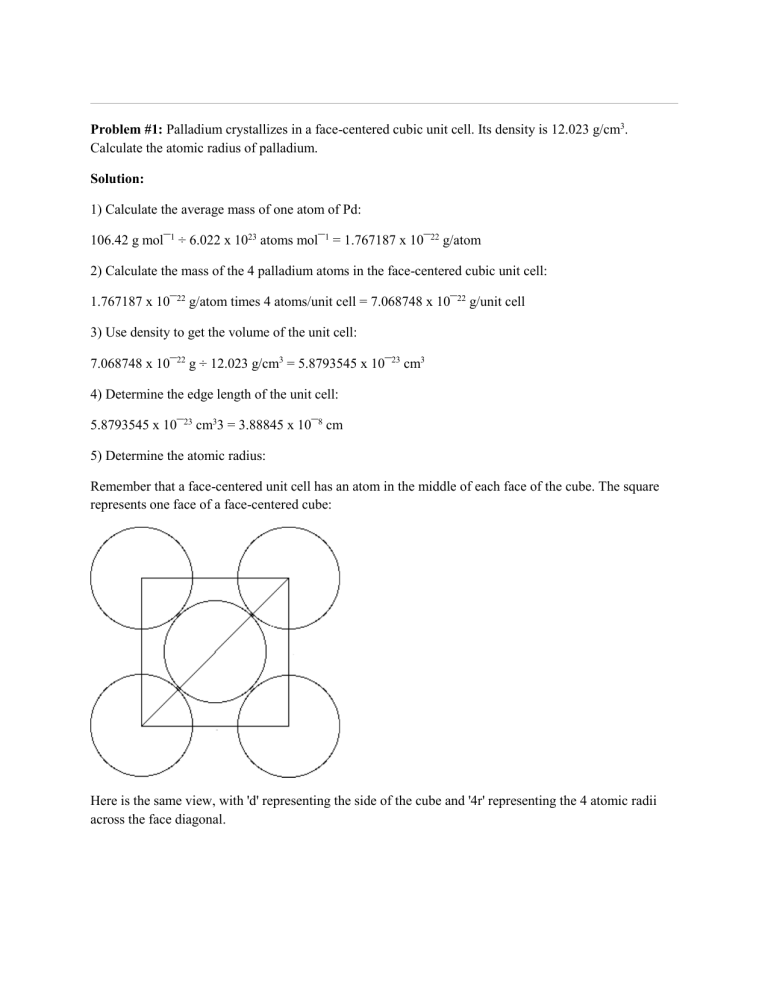
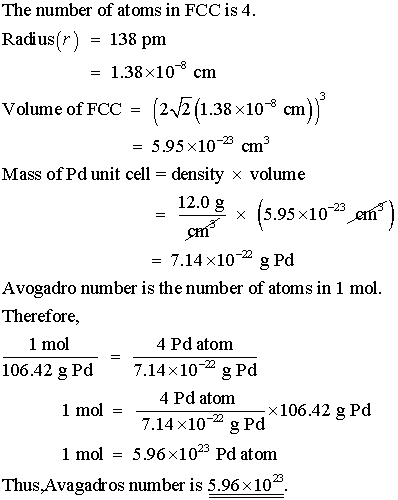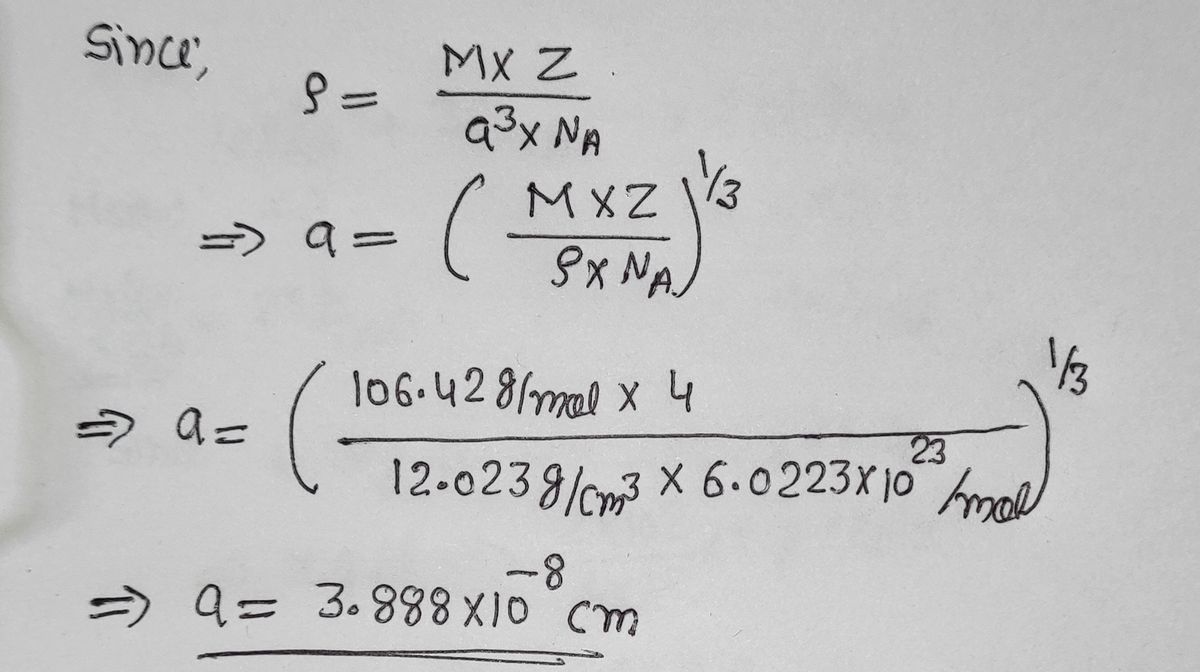Problem #1: Palladium crystallizes in a face-centered cubic unit cell Its density is 12023 g/cm 3 Calculate the atomic radius of palladium | Course Hero

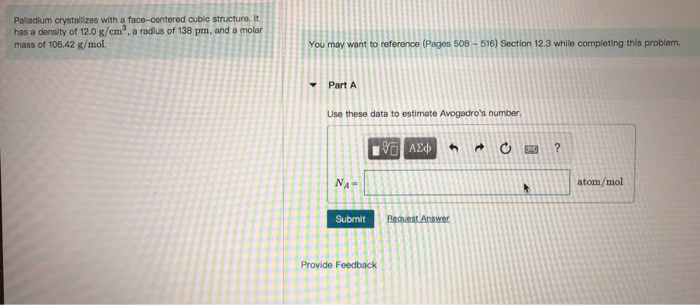
SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's
If the radius of palladium is 248 pm and the lattice type is body centered cubic, what is the - Sarthaks eConnect | Largest Online Education Community

roblem.docx - problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero
SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's number.

SOLVED:Metallic iridium crystallizes in a face-centered cubic lattice, with one Ir atom per lattice point. If the edge length of the unit cell is found to be 382 pm, what is the

SOLVED:**30) Gallium crystallizes in primititre cubic Unit cell The length of the unit cell edge is 3.70 4 The radius ofa Ga atom is 4)7.40 B) 3.70C) 1.85D) 0.930 E) Insufficient data

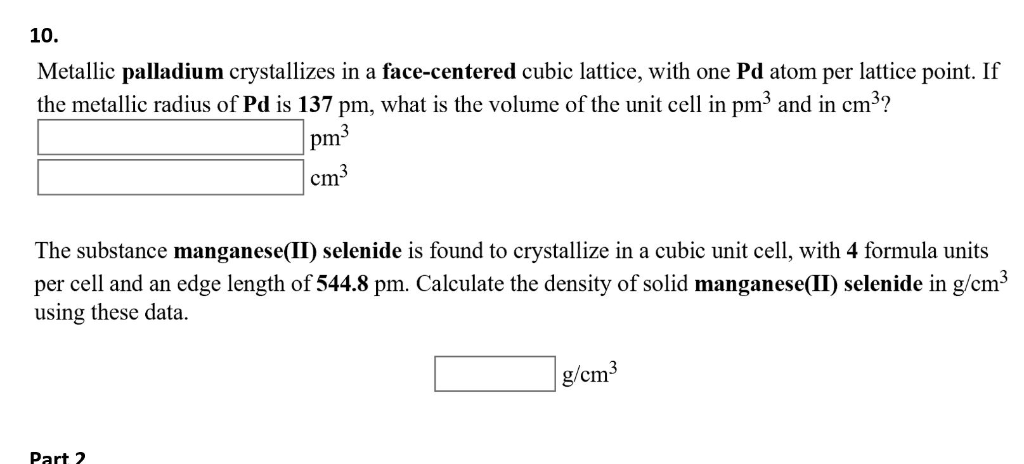
Chapter 3 Homework - Chapter 3 Homework Textbook Problems 9 17 22 25 33 40 44 46 71 79 Additional problems and solutions Problem#1 Palladium | Course Hero

Copper crystallizes in a cubic structure. If the density of the metal is 8.% g/cm^3 and the length of the unit cell edge is 361 picometers, find the number of atoms in

SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's
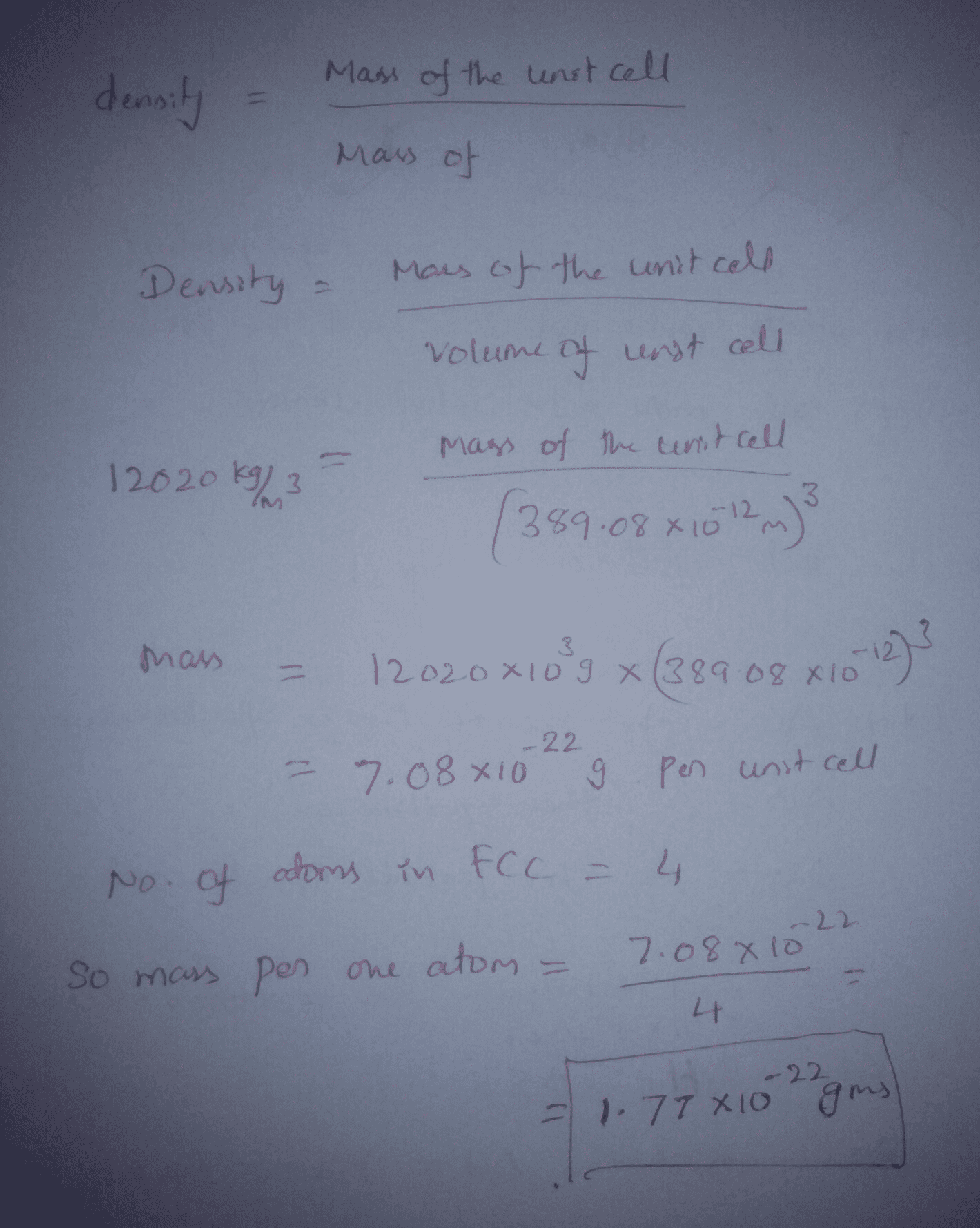
OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

SOLVED:16. Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 glem'. What is the atomic radius of Pd? A. 138 pm B. 1.95 * 10 8 nm C.1.95 * 10-8 cm D. 154 pm E. 0.109 nm
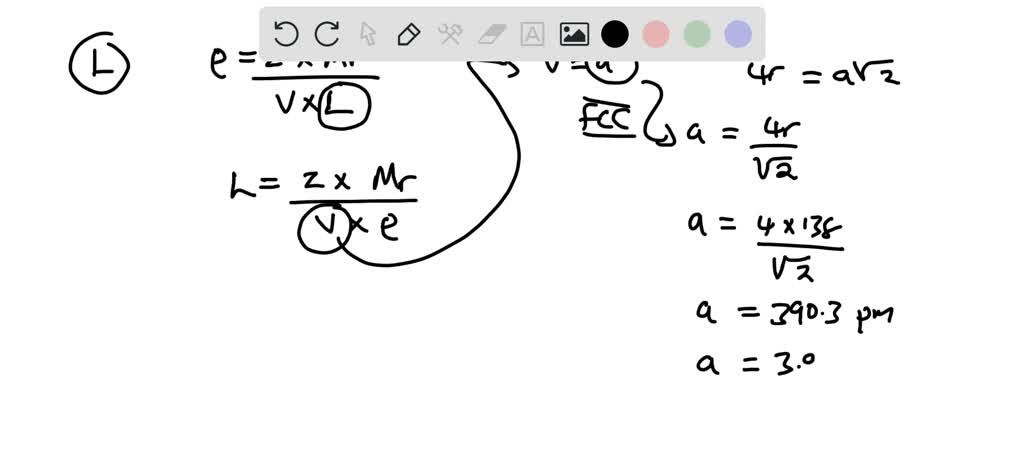
SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of $12.0 mathrm{~g} / mathrm{cm}^{3}$, a radius of $138 mathrm{pm}$, and a molar mass of $106.42 mathrm{~g} / mathrm{mol}$. Use this

SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's